Sysmex goes pink – Let’s fight breast cancer together
Breast cancer is the most frequently diagnosed type of malignancy in women [1]. Yet, early detection via regular screening and genetic testing for one’s predisposition can increase the chances of making this disease curable and, in several cases, avoidable.
Moreover, the application of new molecular diagnostic tools combined with less invasive surgical approaches and personalised treatments have significantly improved the oncological outcome and patient’s quality of life.
We play a significant role in these improvements, offering a broad portfolio of cutting-edge products spanning the entire breast cancer management process.
Breast cancer prevention methods
In most cases, hereditary breast cancer is due to the presence of a deleterious germline (inherited) mutation in BRCA1 and/or BRCA2 genes [2].
Prior to testing, a person will usually have a risk assessment, in which they meet with a genetic counselor or other health care provider to review factors such as which of their relatives had cancer, what cancers they had, and at what ages they were diagnosed. If the assessment suggests that someone has an increased risk of carrying a harmful BRCA1 or BRCA2 gene variant, the genetic counselor can order the appropriate genetic test, if the individual decides to have genetic testing.
Below are the NCCN’s recommendation for BRCA1 and BRCA2 testing [3]:
- Breast cancer diagnosis at the age of ≤45 years
- Multiple breast cancers: bilateral or ipsilateral
- Breast and ovarian cancer at any age
- Male breast cancer
- Triple-negative (oestrogen receptor negative, progesterone receptor negative and HER2/neu negative) breast cancer diagnosed ≤60 years of age
- Pancreatic cancer at any age
- Two or more relatives (≤50 years old) with breast cancer
- Three or more relatives with breast cancer at any age
- A previously identified BRCA1 or BRCA2 mutation in the family
- Personal history of breast cancer between the age of 46-50 and a close family member with breast, ovarian, pancreatic or prostate cancer
- Personal history of breast cancer at any age and a close family member with breast cancer at age 50 or younger, or two or more close family members with breast cancer at any age
- Metastatic prostate cancer at any age
People who inherit such mutations face high risk of developing breast cancer during their lifetime. Once diagnosed with invasive breast cancer, they carry a high risk of developing a second ipsilateral or contralateral breast cancer [4].
Therefore, a correct diagnosis of the presence of such germline mutations combined with suited prevention and screening strategies are of utmost importance to fight such disease.
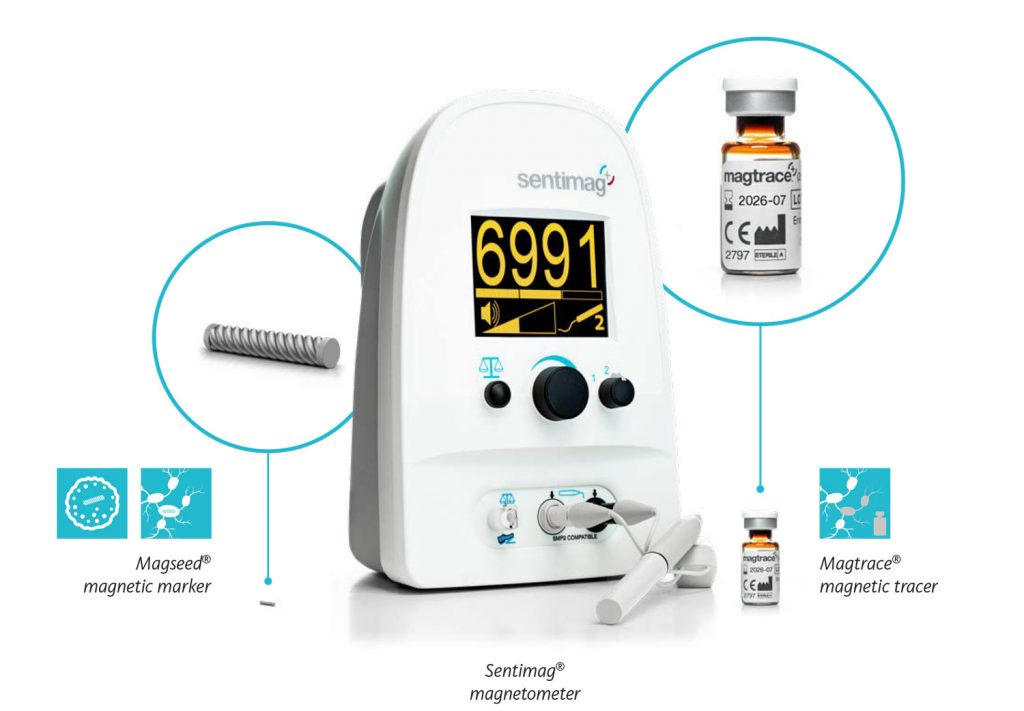
Magtrace® lymphatic tracer for sentinel node detection
Many cancer treatments involve sentinel lymph node biopsy. Sentinel nodes are the first draining lymph nodes and therefore have the highest potential to harbour metastases. Their removal and analysis are essential in determining the nodal stage of the cancer, allowing for an individual treatment plan for the patient.
Magtrace® paramagnetic tracer is a liquid tracer that has been developed specifically for the SLNB procedure which is carried out at the same time as the removal of the suspicious cancer tissue in the breast.
To help identify the sentinel nodes, the tracer is injected into the breast and will migrate through the lymphatic system to the sentinel nodes [5]. With its traceable signal, the nodes containing Magtrace® can be identified using the magnetic sensor in the Sentimag® probe before being removed to determine the spread of the cancer.
Removal of impalpable breast lesions with Magseed®
Upon detection of early-stage breast cancer or a suspicious lump, breast-conserving surgery may be an option – also known as a lumpectomy or partial mastectomy.
This is a surgical procedure that aims to completely remove the lesion and a thin margin of healthy tissue around it to ensure no tumour cells are left behind while conserving as much healthy tissue as possible.
To exactly locate the lesion during surgery, there are a few aids that are used as marker – Magseed® is one of them.
Before the surgery, the Magseed® is deployed in the centre of the lesion using X-ray or ultrasound guidance. The placement can be scheduled whenever convenient for the patient and the hospital staff. Afterwards, this tiny seed of only 1×5 mm in size stays securely in place without causing discomfort to the patient until the day of surgery [6].
During the operation, the Sentimag® system’s probe is used to precisely locate the Magseed® and thereby the lesion in which the Magseed® is placed. This enables the tumour to be removed accurately and completely.
Targeted Axillary Dissection using Sentimag®-Magtrace®/Magseed®
In some cases, the breast cancer treatment plan includes a neoadjuvant chemotherapy – a chemotherapy given prior to surgery. This allows close monitoring of the response, and according to studies, the tumour in the breast can shrink and cancerous lymph nodes can turn from “positive” to “negative” (free of cancer) during the course of the chemotherapy. Thus, the chances increase to receive a less invasive surgical approach afterwards, e.g. breast conserving surgery.
If suspicious lymph nodes are detected in the axilla at the time of diagnosis, they are usually marked before or during the neoadjuvant chemotherapy. For this purpose, a Magseed® marker can be inserted into the suspicious lymph node/s, ensuring accurate identification and removal with the help of the Sentimag® detector during the following surgical intervention.
In addition, a SLNB is also performed using the same instrument, for which the Magtrace® liquid tracer is injected into the breast and migrates to the sentinel nodes. The magnetic sensor in the Sentimag® probe will then detect the signal from the Magtrace® particles and identify the nodes associated [7].
The combination of these two procedures is called targeted axillary dissection (TAD). It has become more common, as first studies have proven it to be more accurate in determining the status of the axilla after chemotherapy and assessing if the patient has down-staged or at best converted to a cancer-free status.
Molecular lymph node assessment with OSNA
Breast cancer typically spreads first to the nearby lymph nodes in the axilla. During surgery, the lymph nodes called sentinel lymph nodes (SLN) are removed and analysed to detect for the presence of metastases – this procedure is called sentinel lymph node biopsy (SLNB).
Depending on the extent of tumour burden present in the SLN, the surgeon decides whether further axillary lymph node dissection is needed or not. Hence, an accurate, reliable and rapid method for the analysis of the SLN is of utmost importance in breast cancer management. SLNB less invasive approach allows to spare many axillary dissections, improving patient’s quality of life.
OSNA is a cutting-edge molecular-based diagnostic test for the accurate and fast staging of lymph nodes that reveals the whole nodal picture, with very high sensitivity. Its clinical value goes beyond the purely lymph node assessment, as it supports personalised treatment decisions and improves patient’s quality of life.
Precision diagnostics for targeted therapy selection in breast cancer – precisely brings to light what needs to be seen
Precision diagnostics are the key to selecting the right therapy at the right time for the right cancer patient.
Gene alterations play a crucial role during the onset and development of breast cancer. Therefore, reliable assessment of the tumour’s mutational status is decisive not only in selecting cancer patients eligible for targeted therapy, but also later in monitoring response to treatment and disease progression.
Nowadays, a range of medications targeting tumour-specific genetic alterations are available and can be prescribed instead of, or in combination with standard chemotherapies, immunotherapies and radiotherapies.
HER2 amplification is seen in approximately 15% of breast cancers and, in the absence of therapy, is associated with a poor prognosis [8,9]. Treatment of patients with HER2 amplification using the monoclonal antibody Herceptin® (Trastuzumab) has been shown to be effective, increasing overall survival time [10,11].
Furthermore, the FGFR1 gene has been shown to be amplified in approximately 10% of breast cancer patients. It indicates a poor outcome, as over-expression of the gene product has been implicated in early relapse [12-14].
Amplification of TOP2A gene is also seen in breast cancer, frequently with co-amplification of HER2 [15,16]. TOP2A gene aberrations are a marker for the response to anthracycline-based chemotherapy in breast cancer patients [12].
To investigate such genetic changes, oncologists can make use of various molecular genetic tests which may support them in making personalised treatment decisions. Sysmex offers a range of products based on cutting-edge molecular biology technologies, supporting health care professionals and patients along the whole pathway of care.
Therapy and recurrence monitoring
Despite surgical and/or systemic primary treatment, up to 30% of breast cancer patients still face disease progression. Thus, monitoring patients for therapy response, particularly resistance mutations and tumour recurrence, is of vital importance to secure the best possible long-term outcome for the patient.
In modern personalised oncology, certain somatic gene alterations have emerged as key biomarkers for disease progression, which are detectable in DNA from tumour tissue or circulating tumour-derived DNA (ctDNA) detected in liquid biopsy. At the stage of monitoring, tissue samples are often not available for analysis. However, liquid biopsies can be sampled repetitively with minimal patient distress and has shown to overcome limitations of tissue biopsies, such as missing tumour heterogeneity, inferior sample accessibility and long turnaround time.
Hence, detection of clinically relevant mutations from a simple blood draw enables oncologists to select the most beneficial therapy and monitor the patient’s response to therapy in real time.
Plasma-SeqSensei™ (PSS) RUO kits are next-generation sequencing-based (NGS) liquid biopsy assays intended for research of actionable mutations relevant in colorectal, NSCLC, breast and thyroid cancers as well as melanoma. Built on ultra-sensitive SafeSEQ technology, the PSS RUO kit portfolio aims to set a new standard for absolute determination of mutant molecules (MMs) independent of real sample DNA input, thus offering researchers a cutting-edge tool to advance personalised oncology. Due to its ability to determine low variant allele frequencies, it is especially useful for minimal residual disease (MRD) detection and other applications requiring sequential measurement like response, recurrence or resistance monitoring.
* Plasma-SeqSensei™ BC RUO Kit is for Research Use Only. Not for use in diagnostic procedures.
- Sung H. et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin., 71(3):209-249.
- Paluch-Shimon S. et al. (2016). Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines. Ann Oncol., 27 (5): v103-v110.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian. V3.
- Kotsopoulos J. (2018). BRCA Mutations and Breast Cancer Prevention. Cancers, 10(12), 524.
- Pedro L. et al. (2019): Impact of locally administered carboxydextran-coated super-paramagnetic iron nanoparticles on cellular immune function. Small, 15, 1900224.
- Kühn F. et al. (2020): A German study comparing standard wire localization with magnetic seed localization of non-palpable breast lesions. In vivo, 34, 1159-64.
- Pohlodek K. et al. (2019): Localization of impalpable breast lesions and detection of sentinel lymph nodes through magnetic methods. Eur. J. Radiol., 120, 108699.
- Tsai-Pflugfelder M, et al. Proc Nat Acad Sci 1988;85:7177-81.
- Bofin AM, et al. Cytopath 2003;14(6):314-9.
- Fountzilas G, et al. BMC Cancer 2013;13:163.
- O’Malley, et al. J Natl Cancer Inst 2009;101(9):644-650.
- Letessier A, et al. BMC Cancer 2006, 6:245.
- Courjal F et al., Cancer Res 1997;57(19):4360-7
- Turner N et al., Cancer Res 2010;70(5):2085-94
- Bofin AM, et al. Cytopath 2003;14(6):314-9.
- Fountzilas G, et al. BMC Cancer 2013;13:163