The Importance of Thyroid Molecular Testing in Management of Thyroid Nodules
By Dr. Mohamed Hani, Medical Products Specialist at ExpressMed Laboratories - Bahrain
 “DON’T UNDERGO SURGERY WITHOUT ALL THE INFORMATION”… Dr. Mohamed Hani
“DON’T UNDERGO SURGERY WITHOUT ALL THE INFORMATION”… Dr. Mohamed Hani
Thyroid nodules, a common endocrine disorder where the numbers of these cases are showing an upward trend over the last several decades. These occur in women more than men, the risk in both increases by age; where a majority of the nodules are benign and only 4-6% are malignant.
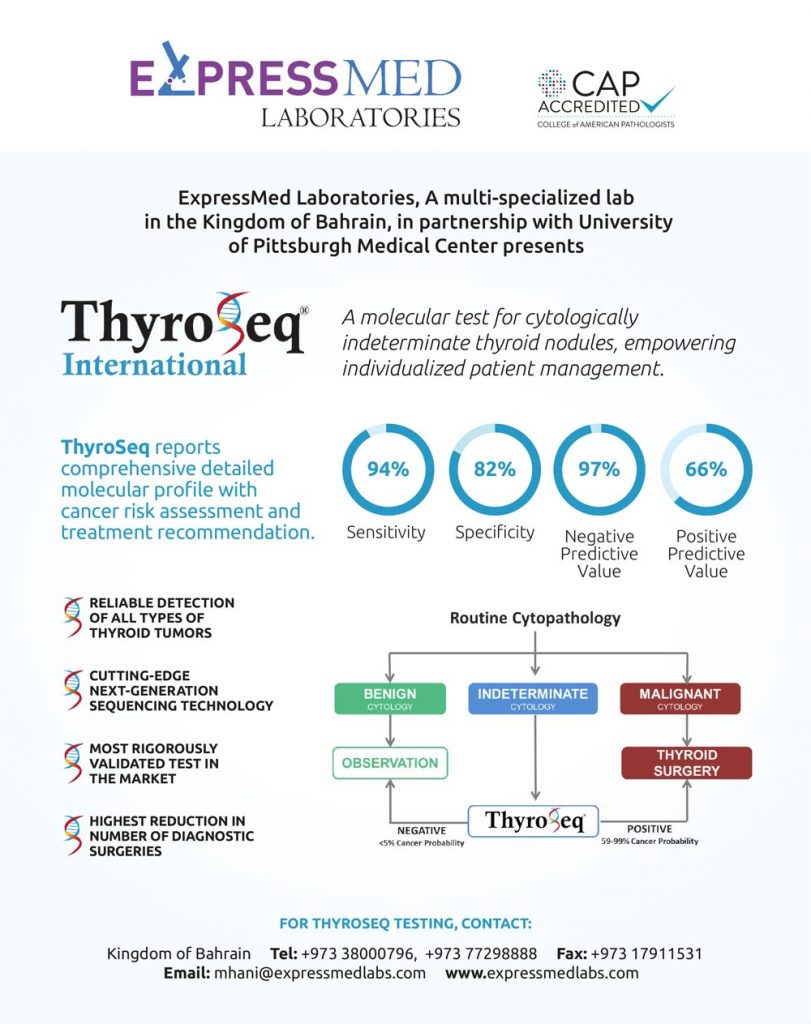
Fine needle aspiration (FNA) is a critical method used in the early stages to evaluate and triage patients with thyroid nodules, 70–75% of cases can be definitively diagnosed by FNA cytology. However, a group of indeterminate lesions (Bethesda III, IV) that accounts for 20% of cases offers a challenge in interpretation and clinical management, which is usually carried out with a cloudy view for the treatment plan. Because of this challenge, many patients may undergo unnecessary surgery leading to the loss of a functioning organ. Molecular testing platforms are highly recognized as an option by the American Thyroid Association (ATA) Guidelines since the year 2015, and it can help identify benignity and establish the diagnosis of cancer in thyroid nodules with high accuracy as well as predict how aggressive the cancer is likely to be, based on the genetic expression in the nodule.
ATA developed evidence-based recommendations to inform clinical decision-making in the management of thyroid nodules and differentiated thyroid cancer. They represent, in our opinion, contemporary optimal care for patients with these disorders.
Academic and Clinical Studies of validating the accuracy of a Next Generation Sequencing assay called ThyroSeq v3 Genomic Classifier, was done by using an extensive series of thyroid samples representing all significant types of thyroid cancers, which also includes Hurthle cell (oncocytic) cancer, like Hurthle cell carcinomas, Hurthle cell adenomas, and hyperplastic nodules with Hurthle cell predominance.
ThyroSeq V3 GC Showed that all types of alterations could be detected preoperatively with a sensitivity of 94%, specificity of 82%, NPV of 97% and PPV of 66% among Bethesda III and IV nodules. It can Interrogates 112 genes for five different classes of molecular alterations: single nucleotide point mutations, insertions/deletion (indels), gene fusions, copy number alterations, and abnormal gene expression.
Along with accepting a number of sample options for molecular testing, such as Thyroid FFPE tissue specimens, FNA cell block, or FNA cytology smear slides; the surprising part is that it is not required to re-FNA if previous FNA smear slide is available! This enables better management of the diagnosis by applying ThyroSeq V3 GC test in practice.
Surely, today we can rely on the results from ThyroSeq V3 GC and provide relief to many patients from undergoing unnecessary surgeries, to provide recommendations for doctors and to determine the most informed treatment decisions for patients with cancer.