Target Controlled Anaesthesia: Where It’s Going and Why.
Natalie Samuda. RM, BSc. Becton Dickinson. Senior Clinical Resource Consultant. Medication Management Solutions. MENAT.
James Waterson. RN, M.Med.Ed. MHE. Becton Dickinson. Medical Affairs Manager, Middle East & Africa.
Samuel Garcia. RN, BHSc. Becton Dickinson. Medical Affairs Director, Europe, Middle East and Africa.
Tina Worth. HV, RGN, LLM, MBA. Becton Dickinson. Medical Affairs Manager, Europe, Middle East and Africa.
In the first article in this series1 we described the fundamental or ‘classic’ models that underpin Total Intravenous Anaesthesia (TIVA) and Target-Controlled Infusion (TCI) anaesthesia such as the Marsh and Schnider Model for Propofol, and the Minto Model for Remifentanil, and briefly introduced new developments. Like all things in medicine our techniques and the tools available to clinicians evolve, and there are also revolutions. In this second piece we will look at the new ‘universal’ Eleveld Model for Propofol and Remifentanil as well as new models for managing special-case patients and for Dexmetomidine, and developments in the delivery methods of TCI medications with allometric scaling, new methods for calculating Lean Body Mass (LBM), and human-factor safety builds.
As a very brief recap, TCI refers to maintaining the desired plasma or effect site concentration of a drug using an infusion pump managed by a microprocessor, and pharmacokinetic (PK) and pharmacodynamic (PD) models. This means that instead of setting a dose-rate on the pump, the pump is programmed to target a required plasma concentration or effect-site concentration. A TCI pump automatically calculates how much drug is needed during induction and maintenance to maintain the desired effect-site or plasma concentration.
A TCI algorithm (the ‘target’ and plan on which the pump relies to deliver appropriate induction and maintenance rates to maintain anaesthesia without overdosing the patient) is based on four processes that occur following injection of any intravenous drug into the body:
Absorption, Distribution, Metabolism, Excretion.
The above are commonly, but not always, affected by weight, and renal and hepatic health. The ‘classic’ models: Marsh for Propofol and Minto for Remifentanil are PK models based on body compartments. ‘Compartments’ relates to theoretical body ‘spaces’ in which a drug is distributed following injection. Conventionally the body compartment that the drug is injected into is V1 (plasma/blood), the next compartment is the ‘vessel-rich’ or ‘fast re-distribution’ compartment and is characterized as V2 (heart, liver etc.). The final compartment, which is anatomically ‘vessel-poor’ and ‘slow’ in terms of re-distribution, is V3 (fatty tissue).
Once a steady state of drug distribution has occurred V1+V2+V3=Vdss where Vdss is the steady-state volume of distribution of the drug.
Of course, drug distribution and the metabolism/elimination of each drug in each compartment also need to be modelled. By convention the rate of elimination of a drug is K10, whilst the movement/distribution between compartments is denoted by K12 (V1 to V2), K21 (V2 to V1), K13 (V1 to V3) and K31 (V3 to V1). If one wants to describe the hysteresis between the time course of plasma concentration and clinical effect, the pharmacokinetic model must be enlarged with a pharmacodynamic part. The link between the plasma and the effect-site is done by using the time constant ke0.
Computer simulations and mathematical modelling of infusion schemes based on the above theories of compartments and clearances give us our models for both Target Plasma Concentration (Cpt) and Target Effect Concentration (Cet) and these can be incorporated into specialist computerised infusion pumps.
TCI pumps deliver the infusion at a constantly altering rate (they alter the rate slightly every few seconds). But it can be useful to think of this one infusion as being a mean-average comprised of three continually calculated infusion rates: a constant rate to replace drug elimination and two exponentially decreasing infusions to match drug removed from central compartment to other peripheral compartments of distribution.
Bariatric, obese, and patients outside a fairly uniform weight-range, as well as pediatric and neonatal patients have previously presented a problem for ‘classic’ TCI models. For example, the Marsh model can significantly overdose obese patients as it does not account for the size of the V3 (fatty tissue) compartment, and the physiological differences between paediatrics and adults has previously required separate models for children with a far higher induction and maintenance doses per kg body weight.
The Eleveld model is, in simple terms, a mathematical synthesis of existing TCI models for both Propofol and Remifentanil. One immediate advantage of using this one model for the two most commonly used medications for TCI, Propofol and Remifentanil is that the model can automatically allow for the potentiating effect of the analgesic infusion on the hypnotic and correct infusion rates to target the assigned Cet accordingly. It is close to being a universal model for patients of all ages and weights as it was developed as a PK-PD model for a broad population range, and to cover the anaesthesia needs of most populations.2
However, the Kim-Obara-Egan Remifentanil model captures the widest patient population and can potentially allow Remifentanil TCI in age ranges from 6 months old to 99 years of age, and from 2.5 to 215 kg.3
A very useful feature of a TCI PK Pump when anaesthetists are beginning to work with new models such as the Eleveld model is the ability to automatically pause the pump after induction to allow for assessment of the patient. Assessments such as questioning a patient after beginning Remifentanil, ‘do you feel dizzy?’ at a common Minto model Cet target of 1.5-3 ng/ml can help establish if the patient has a normal sensitivity to the opiate and may help guide the target to be set for induction and maintenance.
In fact, in recent studies, the Eleveld model has been shown to operate in a very similar manner to the Schnider model4 with depth of anaesthesia measured via Bispectral Index (BI) matching those expected in patients anaesthetised using Schnider, but in the case of the new studies, in patients outside of the usual range of the Schnider model.4
One of the Eleveld model’s particular strengths is that older TCI models were developed using data from very narrow populations in terms of age and weight. But patients who received Propofol TCI with the Eleveld model, titrated to a BI of 40 to 60 (See Table One) from a diverse patient population showed a level of precision for the model of less than 30% divergence from expected BI to Cet in children, adults, and in obese adults. The level of precision was close to 10 BIS units in all the patient groups. This is strong evidence for the Eleveld Propofol Model being applicable as a near-universal TCI model.
Table 1: Bispectral Index Scores versus clinical state.
The Eleveld also showed better prediction of actual measured Propofol plasma concentration in a general population of fifty patients undergoing surgery than either the Marsh or Schnider models in one recent study.5 (See Figure One). The authors suggested that this may be due to the simple fact that the Eveveld model’s creation incorporated more than 15,000 Propofol concentrations from more than 1,000 patients and 30 studies, making the results both robust and generalisable, and because the model includes covariates such as age, weight, height, and sex to predict arterial Propofol concentrations.5
The development of the Hanivoort and Colin6 TCI model for Dexmetomidine has interesting potential for those patients who undergo anaesthesia and are then transferred to the Intensive Care Unit, still intubated, for the recovery period, and for other specialist applications. The medication has long been recognised as a useful treatment where weaning from sedation and mechanical ventilation are anticipated to be difficult, as it is argued that the sedation induced by dexmedetomidine is more akin to natural sleep than that given by other sedative agents, and that it causes less respiratory depression than other agents. The medication also produces spinal and supraspinal analgesia, which aids readiness for extubation due to an opioid-sparing effect. The use of Dexmedetomidine has been associated with a reduced time to extubation compared with midazolam. In one study7 the median time to extubation was 1.9 days less in Dexmedetomidine-treated patients compared with those who received Midazolam (3.7 days [95% confidence interval [CI], 3.1 to 4.0] vs 5.6 days [95% CI, 4.6 to 5.9]; P = 0.01).
A separate meta-analysis8 showed further evidence of economic and clinical benefits of a dexmedetomidine-based sedative regimen. This analysis indicated that dexmedetomidine was associated with a reduction in both length of critical care stay -0.79 days [-1.17 to -0.4], P < .001) and time to extubation (-2.74 hours [-3.8 to-1.65], P < .001; 21.65 vs 23.8 hours, P=.005).
Neurological monitoring for the depth of anaesthesia when using TCI Dexmetomidine should be undertaken using the Modified Observer’s Assessment of Alertness (MOAA) rather than BI as the action of the medication on consciousness is markedly different to other sedatives and hypnotics.

Table 2: The Modified Observer’s Assessment of Alertness/ Sedation Scale (MOAA/S).
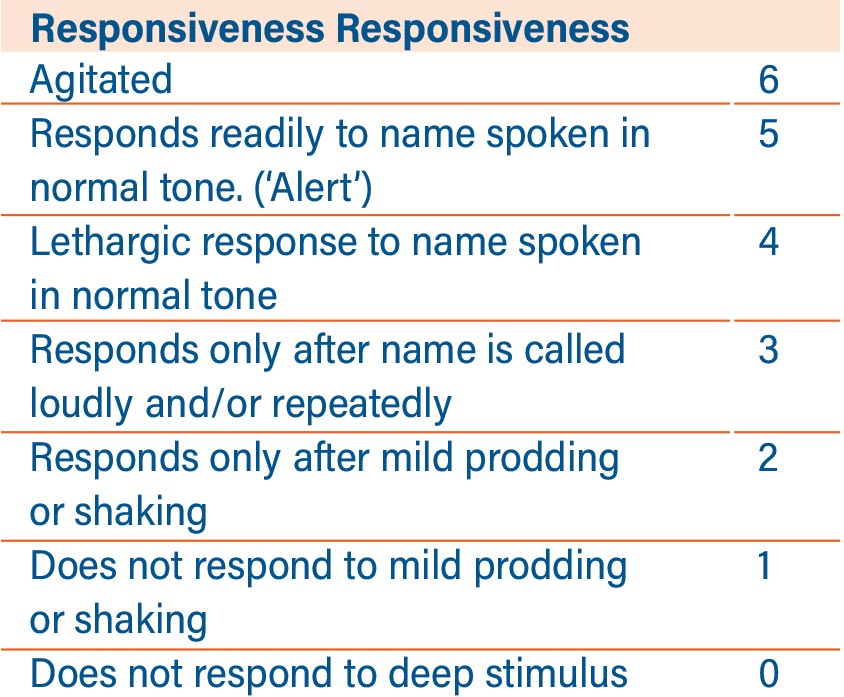
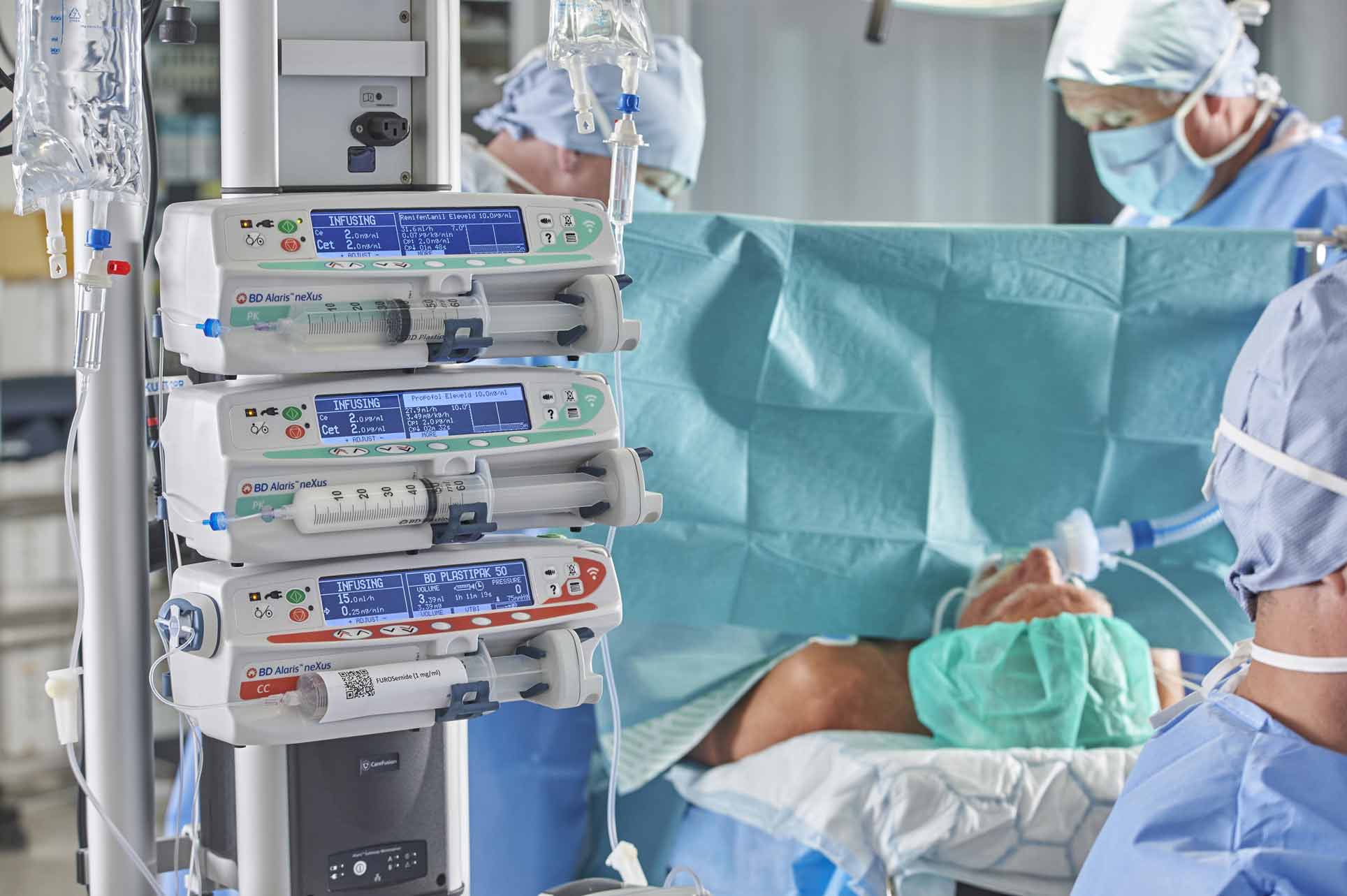
TCI is, of course, much more than abstract modelling. New developments to assist the anaesthetist in maintaining safety whilst maintaining efficiency in the operating room are also available in new generation PK TCI pumps. We discussed in our earlier article how an ideal TCI infusion system or pump should have a large, clear display carrying critical information such as decrement time, current Cet or Cpt and respective targets, current dose rate and concentration and type of agent being infused on one screen that is easy to navigate during the many critical phases of anaesthesia. Equally important is to remember the human factors involved in high-pressure situations. Allometric scaling, where the height, age and weight of children undergoing anaesthesia are automatically correlated against ‘norms’ derived from World Health Organization growth charts9 can help prevent inadvertent and potentially dangerous clinician input on weight and height: from which the target dose will ultimately be derived.
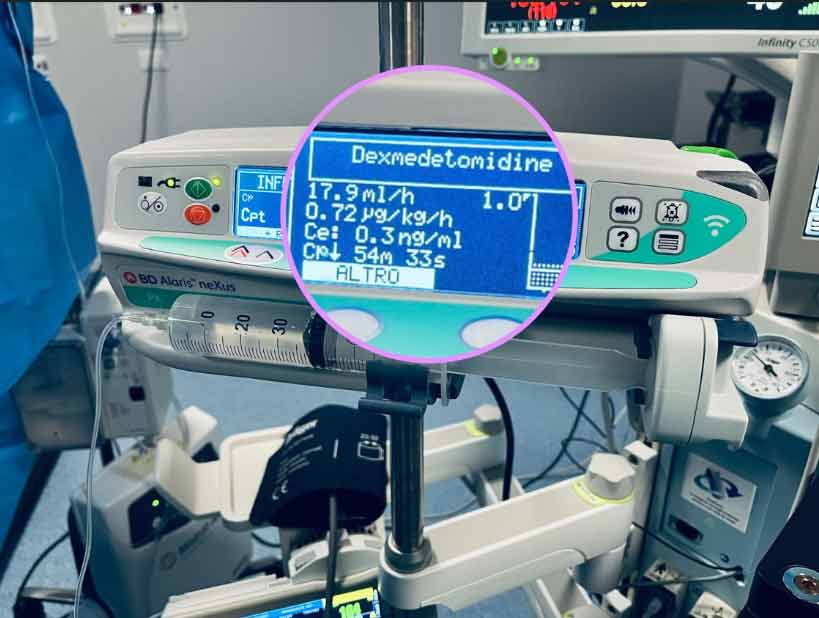
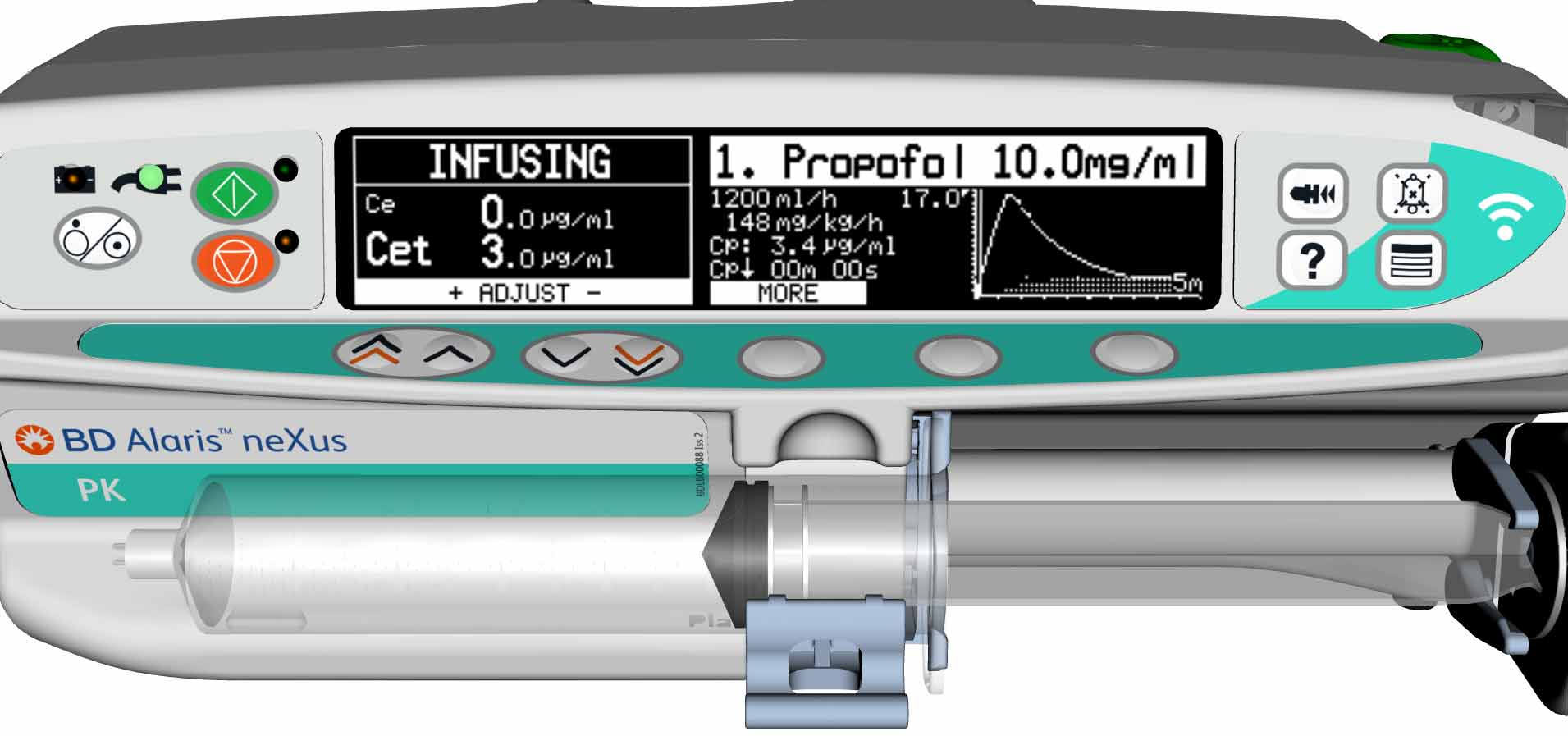
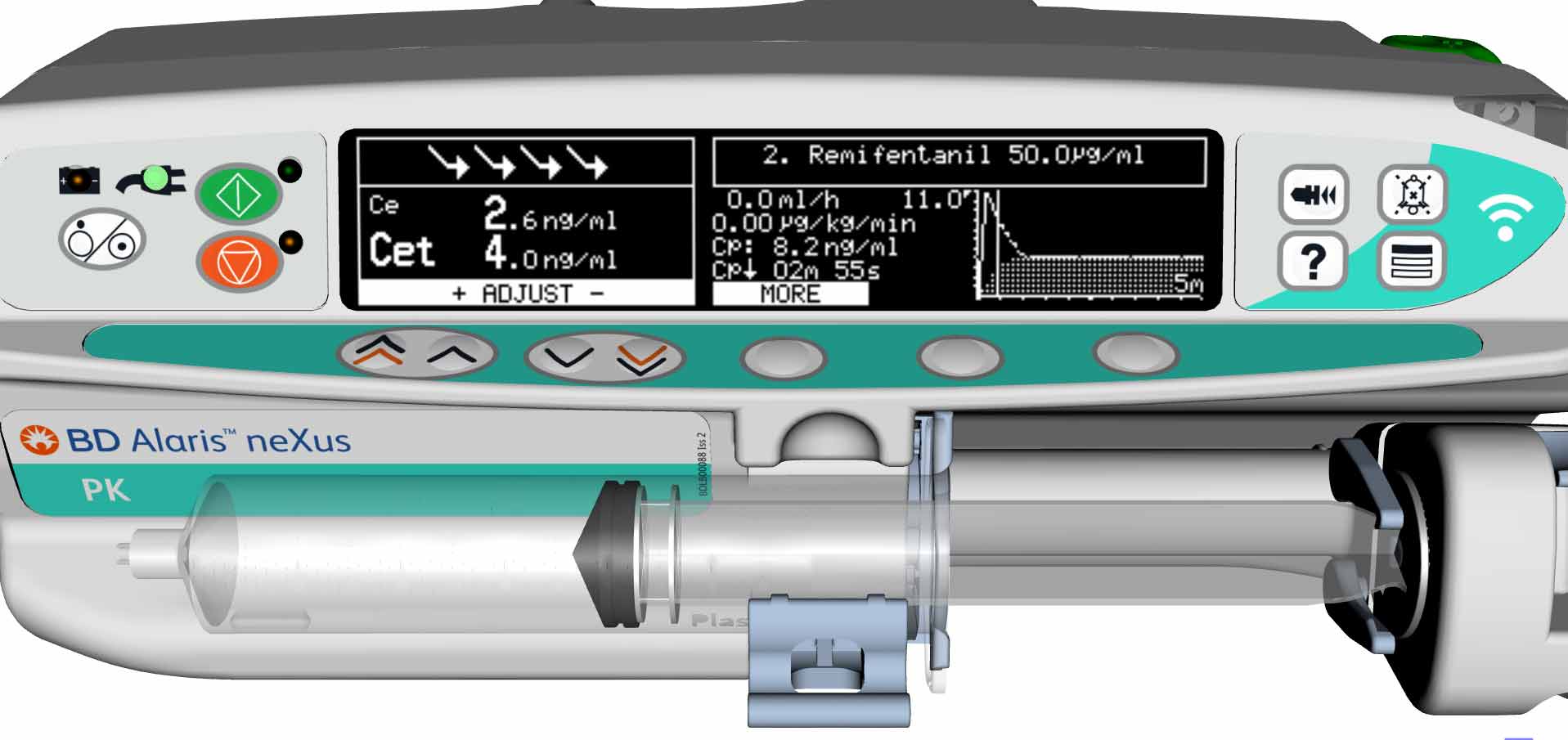
‘Performance level slips’ are a well verified issue in critical care10 and the safety gate of guiding and informing program steps with visual indicators as in a classical Failure Mode and Effect Analysis (FMEA) process has been recognised as a useful preventive tool for medication set-up errors.11 An example in TCI and PK pumps of this human factor guidance is the presentation of Propofol as the infusion agent as black lettering on a white background on the pump’s display – the opposite of the presentation of Remifentanil on the pump and ‘matching’ the physical appearance of the medication itself.
TCI is a ‘mature’ therapy. The new models: Eleveld, and Kim-Obara have substantially added to the useability TCI models for diverse patient groups, and new methods of usage for an established medication has been established by the Hanivoort-Colin model.
There are, of course, target controlled models for other groups of medications such as antibiotics and muscle-relaxants which can potentially add much to precision medicine and evidence-based medicine, but another aspect of intravenous anaesthesia is of growing importance: the environmental impact of healthcare related Greenhouse Gases (GHG). A 2021 paper12 concluded that in our current climate crisis sustainable healthcare initiatives need to look at the impact of inhaled anaesthetics both on the wider environment and on the direct occupational environment of Health Care Workers (HCP). This was essentially a call to action to judiciously consider in each treatment how, when, and why volatile agents such as nitrous oxide (N2O) and desflurane are unavoidable, and whether total intravenous or local-regional anaesthesia could be efficacious substitutes, and to invest in the development of new technologies. Evidence-based medicine can be briefly defined as ‘doing the right thing in the right way’, modelling anaesthesia from evidence, and aspiring to reduce healthcare’s environmental impact must logically be a part of that paradigm.
References:
1. Waterson J, Samuda N. TCI Anaesthesia: New and More Universal Models. Arab Hospitals Magazine. January 2022. https://hospitalsmagazine.com/medtech/tci-anaesthesia-new-and-more-universal-models/
2. D.J. Eleveld, P. Colin, A.R. Absalom, M.M.R.F. Struys. Pharmacokinetic–pharmacodynamic model for propofol for broad application in anaesthesia and sedation. British Journal of Anaesthesia, Volume 120, Issue 5, 2018, Pages 942-959,
ISSN 0007-0912, https://doi.org/10.1016/j.bja.2018.01.018
3. T.K. Kim, S. Obara, T.D. Egan. Disposition of Remifentanil in Obesity A New Pharmacokinetic Model Incorporating the Influence of Body Mass. Anaesthesiology, Vol. 126, Issue 6, 2017, Pages 1019-1032 https://doi.org/10.1097/ALN.0000000000001635
4. Vellinga R, Hannivoort LN, Introna M, Touw DJ, Absalom AR, Eleveld DJ, Struys MMRF. Prospective clinical validation of the Eleveld propofol pharmacokinetic-pharmacodynamic model in general anaesthesia. Br J Anaesth. 2021 Feb;126(2):386-394. doi: 10.1016/j.bja.2020.10.027. Epub 2020 Dec 13. PMID: 33317804.
5. Hüppe T, Maurer F, Sessler DI, Volk T, Kreuer S. Retrospective comparison of Eleveld, Marsh, and Schnider propofol pharmacokinetic models in 50 patients. Br J Anaesth. 2020 Feb;124(2):e22-e24. doi: 10.1016/j.bja.2019.10.019. Epub 2019 Dec 2. PMID: 31806207.
6. Colin PJ, Hannivoort LN, Eleveld DJ, Reyntjens KMEM, Absalom AR, Vereecke HEM, Struys MMRF. Dexmedetomidine pharmacokinetic-pharmacodynamic modelling in healthy volunteers: 1. Influence of arousal on bispectral index and sedation. Br J Anaesth. 2017 Aug 1;119(2):200-210. doi: 10.1093/bja/aex085. PMID: 28854538.
7. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489-499.
8. Pasin L, Greco T, Feltracco P, et al. Dexmedetomidine as a sedative agent in critically ill patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(12):e82913. https://doi.org/10.1371/journal.pone.0082913
9. https://www.who.int/tools/child-growth-standards/standards/weight-for-length-height
10. Rothschild JM, Landrigan CP, Cronin JW, Kaushal R, Lockley SW, Burdick, et al. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med 2005 Aug;33(8):1694-1700. [CrossRef] [Medline]
11. Waterson J, Al-Jaber R, Kassab T, Al-Jazairi AS. Twelve-Month Review of Infusion Pump Near-Miss Medication and Dose Selection Errors and User-Initiated “Good Save” Corrections: Retrospective Study. JMIR Hum Factors. 2020 Aug 11;7(3):e20364. doi: 10.2196/20364. PMID: 32667895; PMCID: PMC7448173.
12. Gaya da Costa M, Kalmar AF, Struys MMRF. Inhaled Anaesthetics: Environmental Role, Occupational Risk, and Clinical Use. J Clin Med. 2021 Mar 22;10(6):1306. doi: 10.3390/jcm10061306. PMID: 33810063; PMCID: PMC8004846.