Pain is the Fifth Vital Sign, and Pain Management is Vital to Quality Patient Care
Ece Aydinc. RN. Clinical Resource Consultant. Becton Dickinson, Medication Management Solutions. Turkey.
Natalie Samuda. RM, BSc. Senior Clinical Resource Consultant. Becton Dickinson, Medication Management Solutions. Middle East, North Africa, and Turkey.
Lisa Gangol. RN, BSN. Senior Clinical Resource Consultant. Becton Dickinson, Medication Management Solutions. Middle East, North Africa, and Turkey.
James Waterson. RN, M.Med.Ed. MHEc. Medical Affairs Manager. Becton Dickinson, Medication Management Solutions. Middle East and Africa.
Background
The relief of suffering is central to the role of every clinician, freedom from pain is crucial to the quality of life of patients and an optimized and safe pain control strategy is an essential part of the patient experience in every healthcare encounter.
A major problem with pain is that it is incredibly diverse. It can range widely in intensity, quality, and duration and has diverse pathophysiologic mechanisms and meanings. As healthcare students we are often told, ‘pain is what the patient says it is’, and this is a useful starting point because pain is very hard to define. As the International Association for the Study of Pain (IASP) states, it is ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.’1 Of course, many patients including those most in need of pain control cannot actually ‘say what it is’, including those with debilitated by disease, those who are too young to be able to vocalize their needs, and those who are too fearful of the consequences of pain therapy itself, and how it might limit their freedoms and ability to engage in society to reveal their suffering.
Therefore, defining the concept of pain in a concise and precise manner presents a challenge, and its diversity and presence in virtually every discipline across healthcare means that we need to employ multiple approaches to its detection, management and to the evaluation of the effectiveness and safety of the strategies we deploy. Nothing in healthcare is risk-free, and every intervention has a risk-benefit offset. The benefits of effective analgesia for acute pain in avoiding the development of chronic pain2 are well-documented. Furthermore, when post-operative pain is not well managed a substantial number of patients will go on to develop chronic postoperative pain, impaired function, delayed recovery from surgery, reduced quality of life, prolonged opioid use, and increased medical costs.3 The increased medical costs are commonly directly attributable to complications of poor mobility including iatrogenic pneumonias and venous thrombosis.4 In complex patients undergoing surgery there is evidence that specific analgesia therapies such as epidural infusion, combined with pharmaceutical and mechanical prophylaxis may substantially reduce the risk of post-operative thromboembolic events.5
As noted above, all therapies have a risk-benefit offset. Much of the risk from Patient-Controlled Analgesia (PCA) and Patient Controlled Epidural Analgesia (PCEA) comes from ‘simple’ programming errors during the set up of the therapy, but there are risk-mitigation technologies which, when integrated into well-constructed Failure Mode Effect Analysis (FMEA)6,7 processes, can reduce this risk. Smart PCA pumps with dose-error reduction software that can alert clinicians of unsafe dose settings and potential programming errors and reporting software from smart PCA pumps can help teams improve the efficacy of the analgesia provided and improve safety.
Extending into homecare, in palliative care and the management of chronic pain the general rule still remains, By the Clock, By the Mouth, By the Ladder8 but when we reach the top of the analgesic ladder or when oral administration is no longer possible and pain control needs to be continuous and escalated by patient demand or bolus then there is a definite place for parenteral therapy (either intravenous or subcutaneous routes) in strategies to relieve suffering and to maximize patients’ social productivity. Indeed, there is mounting evidence that early introduction or ‘anticipatory’ syringe pump usage improves the quality of palliative care.9
Below we will discuss strategies, and the technology that supports these approaches for safer management of pain both in and out of hospital. Below we will review, specifically:
- The use of data from smart PCA and PCEA pumps to assess the efficacy of strategies for pain control.
- Acute post-operative pain management via PCA, and monitoring technology to maintain safety.
- Management of acute post-operative pain and for maternity care via PCEA.
- Regional block analgesia infusions.
- Managing patients in the community via ambulatory PCA and lightweight syringe devices.
Data-driven pain management and strategy reviews and FMEA pump-based protocols:
Pain is a vital sign, in fact pain has been called the fifth vital sign. We measure vital signs via hemodynamic monitoring to obtain the most accurate measures, and we apply numerical scales to pain in order to allow for objective analysis of what is essentially a subjective experience both for the patient (remembering that ‘pain is what the patient says it is’) and for the clinician attempting to rate the patient’s level of pain.10 Bedside clinicians use assessment tools such as the Visual Analogue Scale (VAS), and the Numerical Pain Scale,11 and in patients unable to communicate their distress tools such as Wong-Baker,12 and assessments of physiological data13 to objectively ‘measure’ pain- a truly individual and highly subjective phenomenon.
Pain assessment uses tools such as the VAS and Wong-Baker, and this can be enhanced by physiological data
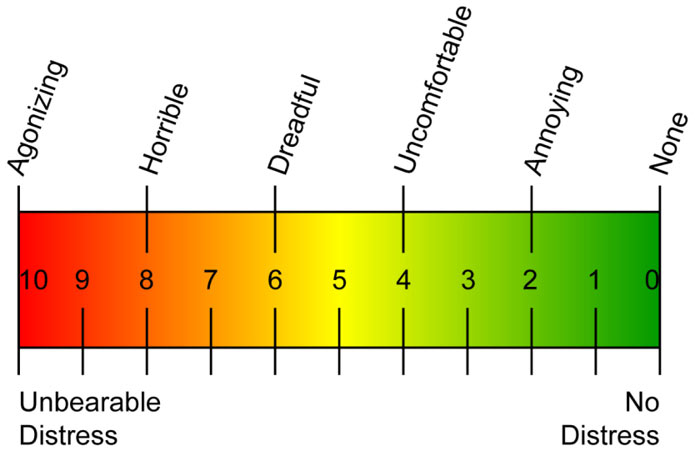
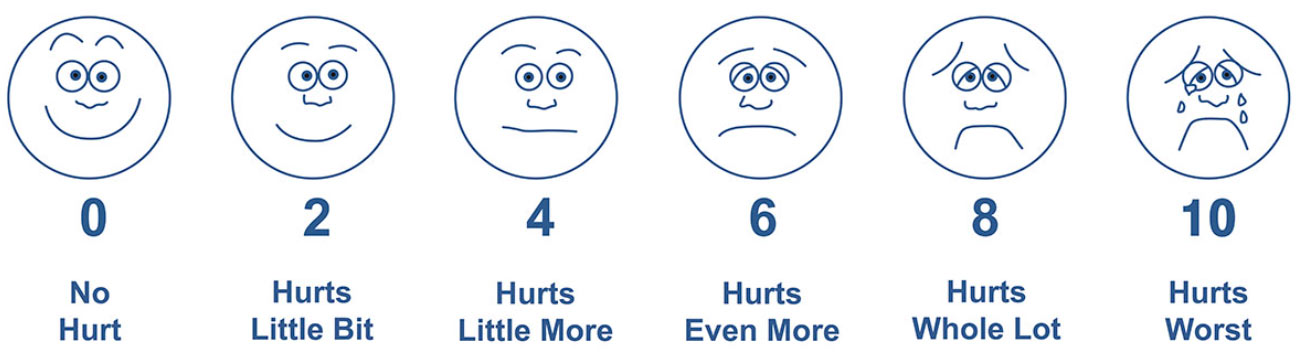
Clinicians can compare their assessments of the patient’s pain and activity to the patient’s analgesia history as presented on PCA and PCEA smart pumps.14 This history should include, at a minimum, the information given in Table 1.
Table 1: Typical PCA values and observations for adults and pediatrics
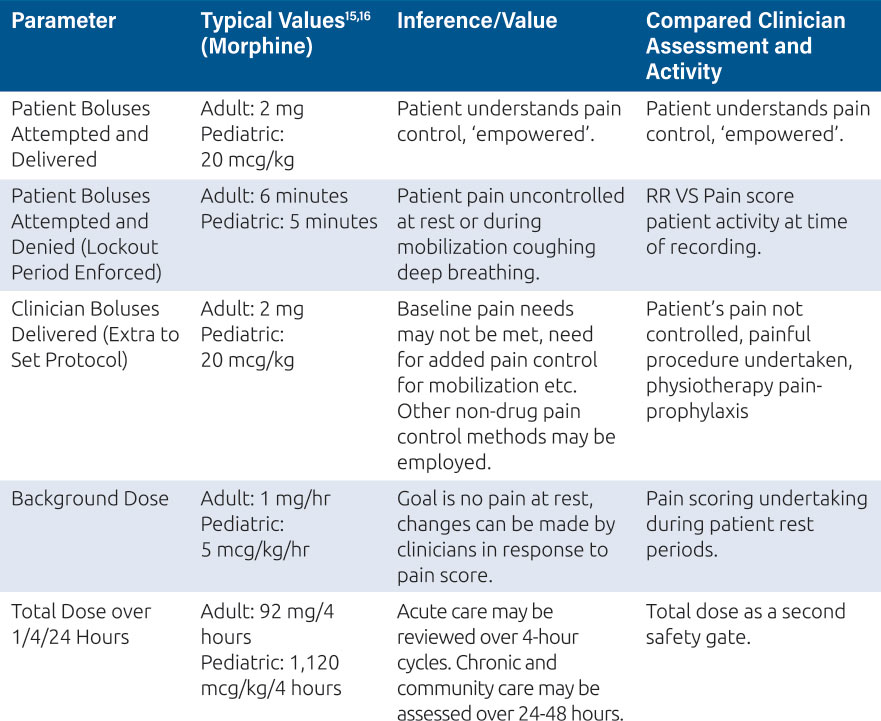
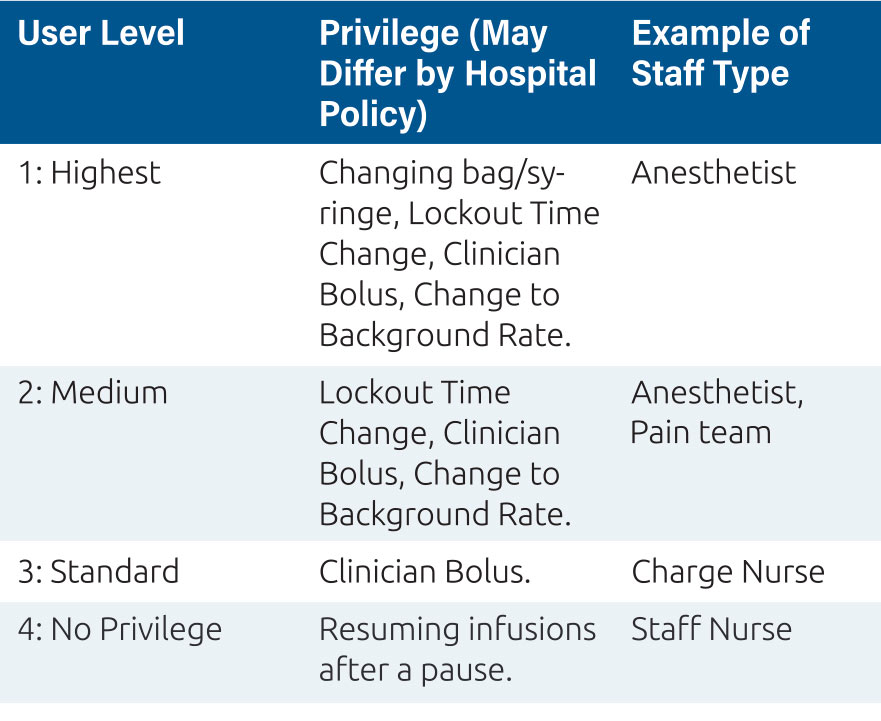
This process allows the clinicians to decide if the patient’s pain needs are being met or if the pain control regimen needs to be altered to ensure effective analgesia for patient recovery, mobilization, rest, comfort and social engagement. The PCA or PCEA pump presents this information on the screen for immediate review and action, for example a pain score of 7 and no patient demands being recorded may indicate a misunderstanding from the patient over how to activate this extra dose, and a clinician administered bolus might be indicated, as well as re-education of the patient. Equally, if the pump history shows several unmet patient demands, where the patient has requested a dose from the pump during the lockout period, and a high pain score this might require an immediate clinician delivered bolus, and changes to background rates and lockout times. These features; boluses and regimen changes are commonly protected by user codes or ‘privileges’ which only certain individuals in the organization can access and these privileges should be defined in the FMEA plan7. For PCA and PCEA devices, a privilege system might look like the one represented in Table 2.
Table 2: Privileges are a key component of the FMEA plan to deliver safe pain therapy
We might view these immediate changes to regimens and the clinicians’ responses to the pain score and the analgesia pump history as pain tactics. The fact that Smart PCA and PCEA pumps retain vast amounts of information from multiple patient events in their event logs means that we have rich data17 from which we can begin to form, or reform, pain strategies and to review the effectiveness of our initial planning to meet patient needs. Pain score recording has also moved, in many facilities, into the patient’s Electronic Medical Record (EMR), and we can use data analysis to map via entity resolution processes18,19 these recordings (and patient activity at the time of scoring) to the analgesia regimen deployed via the pump event log. On a philosophical level this is vital, as in true real world evidence creation we focus on multiple patient events, and on having substantial volumes of data to prove significance.20
At a facility level, we can use the same techniques of analysis on a broader front to interrogate the data for the specific regimens we have put in place against subsequent clinician interventions that have been required to manage patients’ pain needs. For example, we might see a substantial number of clinician boluses being used or increases to background rates or reductions in lockout times being required. This may indicate that our established baseline pain care strategies need review. They may be too conservative overall or do not meet the evolving needs of changes to patient management such as earlier mobilization and changes in physiotherapy approaches for post-surgical patients.21,22
Such extensive data can also help address difficult questions such as whether larger patients, particularly bariatric patients, would benefit from mg/kg/hr dosing and mg/kg bolus doses via their PCA instead of a more standard mg/hr regimen. Weight-based dosing has become the standard in pediatric care.16
Children can benefit from weight-based PCA regimens; education of the child and family and the use of an appropriate assessment tool such as FLACC or COMFORT for children unable to state their needs is vital
As we learn more about regional pain management, a review of strategies including optimal background rates, and whether patient boluses have value can also be tackled.
A retrospective review of data and scoring against pain charts could also enable us to more fully evaluate larger questions, such as the question of the effectiveness of PCA and PCEA ‘auto-bolus’ regimens. We discuss both these techniques in more detail below. For a considerable amount of time, we have been using ‘big’ data generated from general smart pumps to improve medication safety strategies, it is now time to apply the same techniques to improve patient comfort and satisfaction, whilst maintaining the safety of acute pain control.
Regional Analgesia Infusion
In terms of delivering analgesia to meet acute pain needs both peri-operatively and post-surgery, Regional Nerve Block infusion analgesia delivered via smart analgesia pumps is beginning to show significant advantages over general anesthesia and traditional peri-operative and post-operative analgesia regimes. In one recent study, patients undergoing upper limb surgeries showed significantly higher satisfaction scores than those who received general anesthesia23 and the technique has been preferred at other centres for lower limb surgery as it has lower operating room duration per case than general anesthesia24 with comparable results for pain control as general anesthesia.
In terms of patient safety, the technique can be useful for patients who might be considered to be high risk for respiratory depression, but as we will see below there are ways of protecting patients from the effects of opiates whilst maintaining one of the key attributes of PCA therapy: patient mobility.
PCA Monitoring with continuous End-Tidal Carbon Dioxide (EtCO2) Monitoring
Opioid analgesia remains the primary pharmacologic intervention for managing pain in hospitalized patients; however, as with any medication, opioids can cause adverse effects. Unintended advancing sedation and respiratory depression are among the most serious.25
The Joint Commission reported in 2010 that opioid related events resulting in death or permanent loss of function accounted for 0.25% of all events reviewed between 2004 and 2010, and of these 58% of incidents were the result of improper monitoring.26
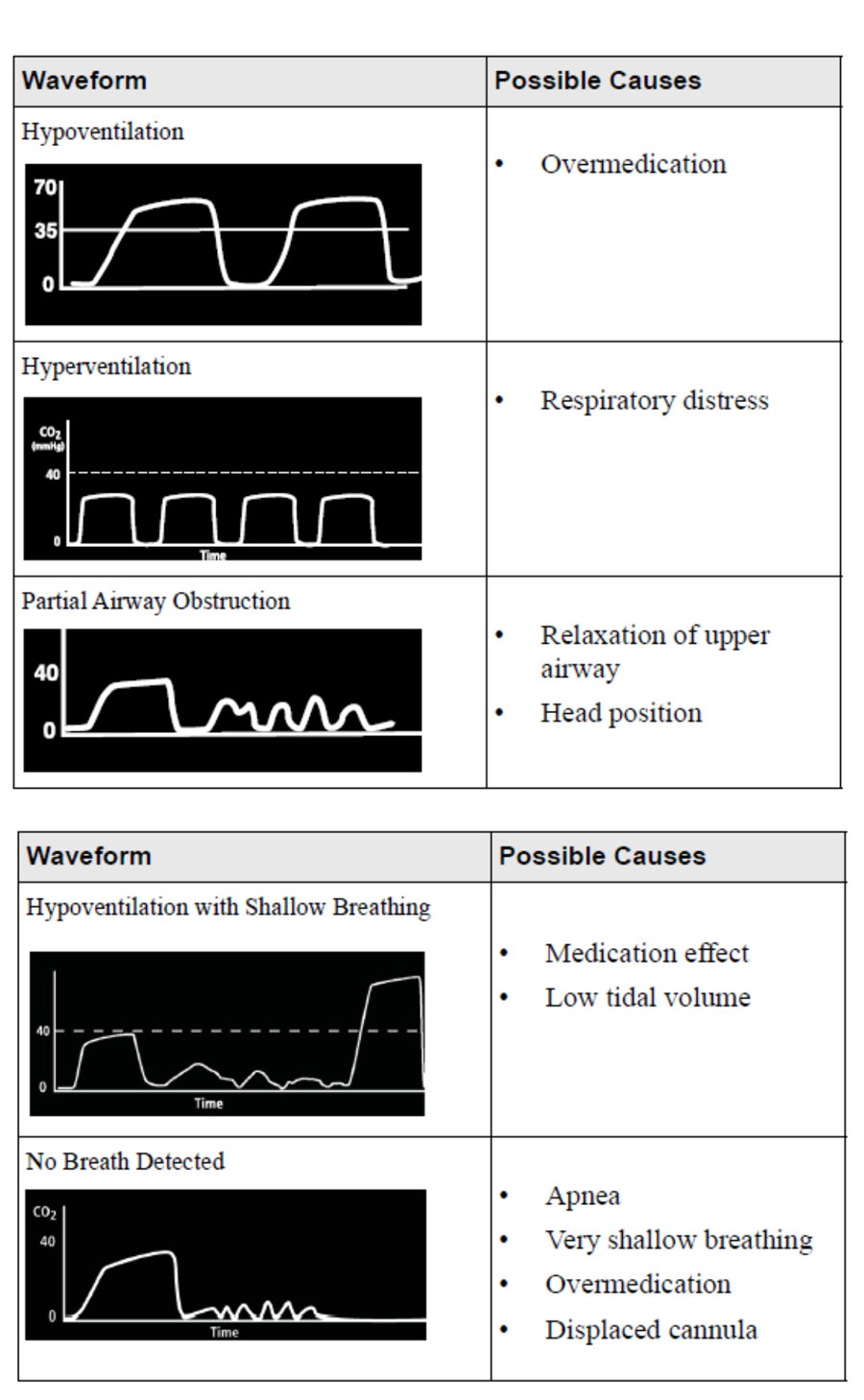
Patient sensitivity to opiates and opioids is hard to predict, and post-operative respiratory failure has been suggested to have multiple determinants.27 Using SpO2 monitoring for impending respiratory failure or depression is problematic as with supplemental oxygen administration the patient may retain acceptable oxygen saturations even while deteriorating. Non-invasive monitoring with an EtCO2 Module on the PCA itself, rather than using wall-mounted monitors allows for patient mobilization, and allows for a ‘pause protocol’, which adds another level of safety. The EtCO2 alarm responds to lowered respiration rates and to hypercapnia to alert the clinician, and automatically pauses the PCA infusion. EtCO2 monitoring also adds to our information about the patient. Rises in respiratory rate, and drops in EtCO2, can correlate with patients experiencing pain. An EtCO2 Module with the option to display EtCO2 readings as a waveform can be used to monitor a patient’s clinical status. Waveform analysis can expand the patient assessment to identify possible cases of overmedication or inadequate pain control. Smart pump alarms can therefore be set to reflect both patient safety concerns and quality of analgesia delivered.
Patients at risk of opioid-induced respiratory depression may benefit from continuous EtCO2 monitoring, the module adds to bedside clinical information about the patients pain state and physiological stability
It may be possible to recoup investment in such technology by a reduction in Critical Care bed usage by high-risk COPD and post-bariatric surgery patients.
Continuous EtCO2 monitoring can be used for early identification of declining physiological status leading to early intervention and could help avoid urgent transfers to critical care.
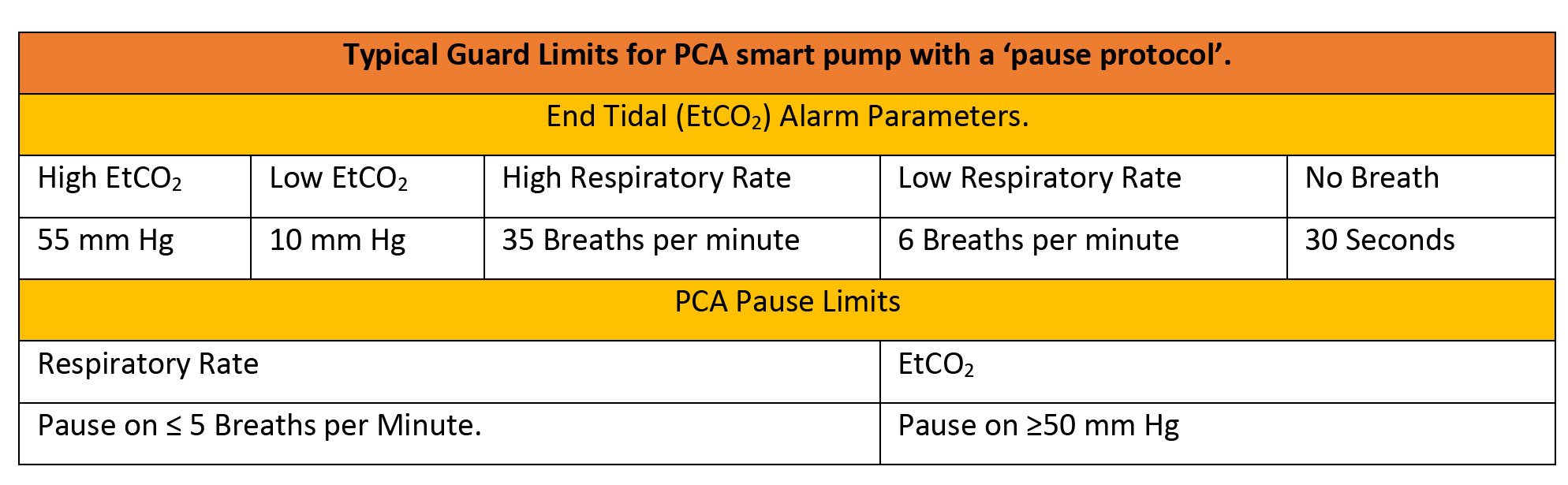
The availability of respiratory and dosing trend data together can improve clinical assessments of patients receiving PCA, leading to patients with better controlled pain and improved safety, while averting costly adverse events.
Typical values for a PCA smart pump with EtCO2 are given below, but all parameters must be editable for specific patient groups.
Epidural management of Acute Pain
To reduce the risk of incorrect connections and injections, the ISO 80369 series of standards was issued in 2016. It defines a separate, incompatible connection for epidural and regional block anesthesia and analgesia.
In simple terms, an intravenous administration line or standard syringe will not fit an NRFit® epidural cannula, and an NRFit® epidural administration set will not fit onto an intravenous cannula.
Epidural administration line safety goes beyond the connector, however. The Institute for Safe Medication Practice and ECRI state that facilities should always, ‘use yellow-lined tubing without injection ports for epidural infusions to set its appearance apart from typical IV tubing, and never use yellow-lined tubing for anything other than epidural administration.’28.
The Faculty of Pain Medicine of the United Kingdom in its ‘Best Practice in the Management of Epidural Analgesia in the Hospital Setting’ recommends the use of yellow tubing to differentiate epidural/spinal lines from arterial (red), enteral (purple) and regional (grey) infusions.29
It is usual for facilities to set pre-programmed epidural therapy protocols for maternity units and for surgical units. Each protocol would specify background rate, lockout time and patient bolus, clinician bolus and PIEB (Programmed Intermittent Epidural Bolus). The protocols are commonly set as total dose per hour, total volume per hour, and can be set as weight-based, by dose/kg.
Extra boluses may be delivered at the clinician’s discretion, with soft and hard dose limits applied to these doses and to the background rate, patient doses and any PIEB settings. A ‘classic’ Patient Controlled Epidural Analgesia (PCEA) maternity unit regimen without PIEB is described below, drawn from a metanalysis of over 2,000 patient events:30
In PIEB the pump delivers an intermittent dose of analgesia on a set cycle, whilst maintaining the background infusion and allowing patient boluses which are regulated by a lockout period. There have been some studies that indicate shorter duration of labor and improved patient satisfaction with PIEB.31
The theory behind PIEB is that the automated bolus improves the spread of anesthetic solution within the epidural space, and therefore enhances the sensory blockade. 32
Smart Epidural Pumps are capable of delivering Programmed Intermittent Epidural Bolus (PIEB) regimens with an intermittent dose of analgesia being delivered on a set cycle, whilst maintaining the background infusion and allowing patient boluses which are regulated by a lockout period


PCA Management Strategies in Palliative care
Palliative care is an interdisciplinary approach that includes specialized medical and nursing care that improves the quality of life of patients (adults and children) and their families when they face problems with life-limiting diseases33. Pain is one of the most frequent and serious symptoms experienced by patients in need of palliative care. Pain is associated with both the disease as well as treatment, and management is essential from the onset of early disease through long-term survivorship or end-of-life care34.
Patient Controlled Analgesia (PCA) is an effective and frequently preferred treatment method at home and in the hospital for the treatment of chronic pain symptoms in palliative care patients.
PCA allows for faster relief of episodic incident and breakthrough pain and can give patients a greater sense of personal control over their pain. The primary indication for PCA is the patient who requires parenteral analgesia due to severe pain and the oral/transdermal/rectal route not being practical, and who has incident pain or other pain patterns that are not predictable.35,36
PCAs which are small, light and mobile can increase the patient’s mobility. Long battery life and adjustable and varied infusion modes are key to meeting the needs of the patient or clinic. The available modes should include continuous only, bolus only, continuous and bolus, and automatic intermittent bolus. When setting the PCA the patient dose interval (minutes of ‘lockout’) should be set in consultation with the patient and carers. The continuous dose is set in either mg/hr or mcg/hr and may be expected to be higher than the doses used in acute care as the patient may have developed tolerance to the medication and has entered a chronic pain cycle. The ‘rate and time limit’ that determines the maximum amount of drug to be dispensed in a given period of time is usually set to provide three to five times the estimated required hourly dose including patient boluses within one-, four-, or twenty-four-hour limits. However, because of the need for frequent dose adjustments in palliative care, this limit may be set with a great deal of ‘elasticity’ by the skilled practitioner. Most palliative care patients will need both PCA demand and continuous infusion dosing.35,36
In addition to intravenous drug administration used to maintain symptom control in pain management, continuous subcutaneous infusion (CSCI) delivered by syringe pump is a useful administration method.37 There is evidence that drug therapy with continuous subcutaneous infusions (CSCI) over 24 to 48 hours have several benefits, both in patient care and in the application of healthcare resources.38
Strategies for palliative care and ambulatory chronic pain regimens can be reviewed and optimized through the mapping of pump-log data to patient self-reports on comfort and effectiveness. The PCA’s safety software, which should also have the ability to be set for weight-based dosing is enhanced in community settings by security systems such as lockboxes as a physical control over accidental or unauthorized modification of the program. Accessories such as carry packs and bags enhance patient mobility and allow for increased social interaction and quality of life.
Palliative care is not just about alleviating symptoms, it should begin long before the patient receives end-of-life care in order for such care to improve their quality of life and enable them to do more, even though their disease is in a palliative stage. Indeed, there is increasing evidence that early administration or ‘anticipated’ syringe pump use improves the quality of palliative care.9
Accessories such as ambulatory bags and lockboxes can improve patient autonomy and allow them opportunities for greater social interaction

Small syringe pumps are used in palliative care where a continuous dose and clinician bolus strategy for pain control is planned
References:
1. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020 Sep 1;161(9):1976-1982. doi: 10.1097/j.pain.0000000000001939. PMID: 32694387; PMCID: PMC7680716.
2. Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. 2010 Dec;11(12):1859-71. doi: 10.1111/j.1526-4637.2010.00983.x. Epub 2010 Oct 28. PMID: 21040438.
3. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017 Sep 25;10:2287-2298. doi: 10.2147/JPR.S144066. PMID: 29026331; PMCID: PMC5626380.
4. Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017 Mar 1;118(3):317-334. doi: 10.1093/bja/aex002. PMID: 28186222.
5. Shouhed D, Amersi F, Sibert T, Sibert K, Hemaya E, Silberman AW. Thromboprophylaxis and major oncologic surgery performed with epidural analgesia. JAMA Surg. 2013 Jan;148(1):81-4. doi: 10.1001/2013.jamasurg.5. PMID: 22987072.
6. Stamatis D. Failure mode and effects analysis: FMEA from theory to execution. Milwaukee, WI: ASQC Quality Press; 1995.
7. Cronrath P, Lynch TW, Gilson LJ, Nishida C, Sembar MC, Spencer PJ, West DF. PCA oversedation: application of Healthcare Failure Mode Effect (HFMEA) Analysis. Nurs Econ. 2011 Mar-Apr;29(2):79-87. PMID: 21667674.
8. Ventafridda V, Saita L, Ripamonti C, De Conno F. WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 1985;7(1):93-6. PMID: 2409039.
9. Bowers B, Pollock K, Dickman A, Ryan R, Barclay S. Anticipatory syringe pumps: benefits and risks. BMJ Support Palliat Care. 2021 Sep;11(3):303-304. doi: 10.1136/bmjspcare-2020-002735. Epub 2021 Jan 19. PMID: 33468505; PMCID: PMC8380901.
10. Grissinger M. Safety and patient-controlled analgesia: part 2: how to prevent errors. P T. 2008 Jan;33(1):8-9. PMID: 19749992; PMCID: PMC2730066.
11. Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001 Dec;8(12):1153-7. doi: 10.1111/j.1553-2712.2001.tb01132.x. PMID: 11733293.
12. Garra G, Singer AJ, Taira BR, Chohan J, Cardoz H, Chisena E, Thode HC Jr. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010 Jan;17(1):50-4. doi: 10.1111/j.1553-2712.2009.00620.x. Epub 2009 Dec 9. PMID: 20003121.
13. Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2016 Jul 16;7(7):CD001069. doi: 10.1002/14651858.CD001069.pub5. PMID: 27420164; PMCID: PMC6457867.
14. Chumbley G, Mountford L. Patient-controlled analgesia infusion pumps for adults. Nurs Stand. 2010 Oct 27-Nov 2;25(8):35-40. doi: 10.7748/ns2010.10.25.8.35.c8067. PMID: 21140774.
15. Taketomo et Al. Pediatric Dosing Handbook. 2008.
16. Drug Doses: Frank Shann- RCH Melbourne. http://www.drugdoses.net/ Accessed 22 July 2022. Also available via iOS and Android Applications.
17. Kuo IT, Chang KY, Juan DF, Hsu SJ, Chan CT, Tsou MY. Time-dependent analysis of dosage delivery information for patient-controlled analgesia services. PLoS One. 2018 Mar 15;13(3):e0194140. doi: 10.1371/journal.pone.0194140. PMID: 29543837; PMCID: PMC5854274.
18. Christen P. Data Matching: concepts and techniques for record linkage, entity resolution and duplicate detection. Springer, Berlin, Heidelberg; 2012.
19. Talburt JR. Principles of entity resolution. Entity Resolution and Information Quality. Morgan Kaufmann; 2011. 1–37.
20. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, LaVange L, Marinac-Dabic D, Marks PW, Robb MA, Shuren J, Temple R, Woodcock J, Yue LQ, Califf RM. Real-World Evidence – What Is It and What Can It Tell Us? N Engl J Med. 2016 Dec 8;375(23):2293-2297. doi: 10.1056/NEJMsb1609216. PMID: 27959688.
21. Guerra ML, Singh PJ, Taylor NF. Early mobilization of patients who have had a hip or knee joint replacement reduces length of stay in hospital: a systematic review. Clin Rehabil. 2015 Sep;29(9):844-54. doi: 10.1177/0269215514558641. Epub 2014 Dec 1. PMID: 25452634.
22. Hu Y, McArthur A, Yu Z. Early postoperative mobilization in patients undergoing abdominal surgery: a best practice implementation project. JBI Database System Rev Implement Rep. 2019 Dec;17(12):2591-2611. doi: 10.11124/JBISRIR-D-19-00063. PMID: 31725070.
23. Suresh P, Mukherjee A. Patient satisfaction with regional anaesthesia and general anaesthesia in upper limb surgeries: An open label, cross-sectional, prospective, observational clinical comparative study. Indian J Anaesth. 2021 Mar;65(3):191-196. doi: 10.4103/ija.IJA_1121_20. Epub 2021 Mar 13. PMID: 33776108; PMCID: PMC7989486.
24. Neal-Smith G, Hopley E, Gourbault L, Watts DT, Abrahams H, Wilson K, Athanassoglou V. General Versus Regional Anaesthesia for Lower Limb Arthroplasty and Associated Patient Satisfaction Levels: A Prospective Service Evaluation in the Oxford University Hospitals. Cureus. 2021 Aug 9;13(8):e17024. doi: 10.7759/cureus.17024. PMID: 34522505; PMCID: PMC8425506.
25. Jarzyna D, Jungquist CR, Pasero C, Willens JS, Nisbet A, Oakes L, Dempsey SJ, Santangelo D, Polomano RC. American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Manag Nurs. 2011 Sep;12(3):118-145.e10. doi: 10.1016/j.pmn.2011.06.008. PMID: 21893302.
26. Joint Commission (2010). Comprehensive accreditation manual for hospitals. Oak Brook, IL: Joint Commission.
27. Johnson RG, Arozullah AM, Neumayer L, Henderson WG, Hosokawa P, Khuri SF. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007 Jun;204(6):1188-98. doi: 10.1016/j.jamcollsurg.2007.02.070. PMID: 17544077.
28. https://www.ismp.org/resources/epidural-iv-route-mix-ups-reducing-risk-deadly-errors
29. https://fpm.ac.uk/sites/fpm/files/documents/2020-09/Epidural-AUG-2020-FINAL.pdf
30. Guo S, Li B, Gao C, Tian Y. Epidural Analgesia With Bupivacaine and Fentanyl Versus Ropivacaine and Fentanyl for Pain Relief in Labor: A Meta-Analysis. Medicine (Baltimore). 2015 Jun;94(23):e880. doi: 10.1097/MD.0000000000000880. PMID: 26061307; PMCID: PMC4616487.
31. George RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg. 2013 Jan;116(1):133-44. doi: 10.1213/ANE.0b013e3182713b26. Epub 2012 Dec 7. Erratum in: Anesth Analg. 2013 Jun;116(6):1385. PMID: 23223119.
32. Riley ET, Carvalho B. Programmed Intermittent Epidural Boluses (PIEB) for Maintenance of Labor Analgesia: A Superior Technique to Continuous Epidural Infusion? Turk J Anaesthesiol Reanim. 2017 Apr;45(2):65-66. doi: 10.5152/TJAR.2017.09031. Epub 2017 Apr 1. PMID: 28439433; PMCID: PMC5396898.
33. https://www.who.int/news-room/fact-sheets/detail/palliative-care. Accessed 22 August 2022.
34. https://doi.org/10.3322/caac.20112 Accessed 22 August 2022.
35. McCaffery M, Pasero C, eds. Pain: Clinical Manual. 2nd Ed. St Louis, MO: Mosby; 1999.
36. Caraceni, Augusto, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. The Lancet Oncology 2012; 13.2:
e58-e68.
37. Dickman A, Bickerstaff M, Jackson R, Schneider J, Mason S and Ellershaw J, Identification of drug combinations administered by continuous subcutaneous infusion that require analysis for compatibility and stability, Dickman et al. BMC Palliative Care. 2017.
38. Baker J, Dickman A, Mason S, Bickerstaff R, McArdle A, Lawrence I, Stephenson F, Paton N, Kirk J, Waters B and Ellershaw J, An evaluation of continuous subcutaneous infusions across seven NHS acute hospitals: is there potential for 48-hour infusions? BMC Palliative Care. 2020.