Specialty Hospital In Jordan Adopts Innovative Blood Clot Prevention Device For High-risk Patients In The Vascular Department
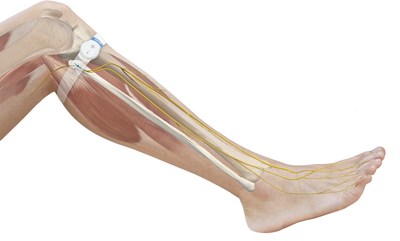
The Specialty Hospital, Amman, Jordan, today announced it is the first hospital within the MENA region to adopt the innovative geko™ device for Venous Thromboembolism (VTE) prevention in high-risk vascular patients, unable to be prescribed standard of care.

VTE is a serious complication for patients in vascular care units and a preventable cause of hospital deaths worldwide[1]. The current standard of care for VTE prevention, when blood thinners cannot be prescribed, due to bleed risk, is a boot-like cuff that compresses the leg to increase blood flow, called intermittent pneumatic compression (IPC). IPC, however, is not suitable for all patients due to vascular disease, fragile skin, or complex limb injury. IPC also requires resource to correctly fit the cuffs and pneumatic pumps are not always readily available. The result is the need for an alternative mechanical intervention when IPC is impractical or unavailable.
The geko™ device on the leg.To address this unmet need, the vascular department at the Specialty Hospital, led by Dr. Walid Mohammed Hasan Masoud, has adopted the innovative geko™ device for blood clot prevention. The small watch-sized device sticks to the side of the knee, increasing blood flow in the deep veins of the calf[2], via painless electrical pulses, at a rate equal to 60 percent of walking[3] without a patient having to move.
Influenced by compelling real-world data, reporting a zero VTE incidence in immobile acute stroke patients[4] , Dr Masoud saw the potential in the use of the geko™ device for blood clot prevention in immobile, high-risk vascular patients. Now in routine use in the unit, Dr. Masoud is leading wider awareness of the neuromuscular electrostimulation device to address other areas of unmet need at the Speciality Hospital, including complications related to swelling after surgery and hard-to-heal chronic wounds (leg ulcers).

Dr. Walid Masoud, senior consultant in vascular, endovascular and kidney transplant surgery at the Specialty Hospital, said: VTE is a complicating factor in the recovery of many critically ill patients within the vascular department setting; and for those unable to be prescribed drug or IPC prophylaxis there is a real danger of serious, sometimes fatal, blood clotting. My team was therefore keen to find an alternative mechanical intervention to ensure that all patients can receive appropriate VTE prophylaxis when standard of care cannot be prescribed.
“We welcomed the opportunity to examine the role of the innovative geko™ device in our vascular care population. Our findings show the geko™ device is safe and well-tolerated and can be used to protect patients until it is safe to prescribe blood-thinning drugs.”

Bernard Ross, founder and CEO of Sky Medical Technology, said: “Vascular clinicians constantly have to weigh an acceptable drug VTE prophylaxis bleeding risk, in acutely or critically ill inpatients, against use of mechanical prophylaxis when a bleed risk is unacceptable. I am proud that the geko™ device can provide an alternative mechanical intervention without bleed risk and delighted Dr. Masoud and his clinical team have embraced this capability and its wider application in pre- and post-operative oedema management and wound therapy.”
References
- Geerts WH, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;1336 Suppl:381s–453s.
- A. Nicolaides, M Griffin. Measurement of blood flow in the deep veins of the lower limb using the geko™ neuromuscular electro-stimulation device. Journal of International Angiology August 2016-04.
- Tucker A, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. The International journal of angiology: official publication of the International College of Angiology, Inc. 2010 Spring; 19(1): e31-7.
- Natarajan I, et al. Poster: The use of the geko™ device and the activation of the foot and calf pumps for prevention of venous thromboembolism in patients with acute stroke. Data on file Firstkind Ltd 2019.
NICE medical technologies guidance [MTG19] Published date June 20 2014.














